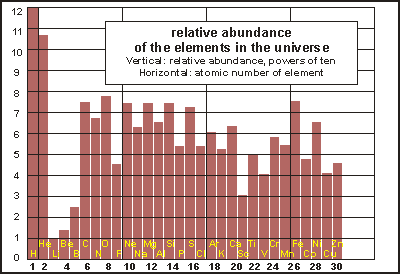
 In
the physical evolution of the planet, the heavier elements became more
abundant as the solar system formed. The accompanying bar chart shows the
relative abundances of the most important elements. Note that the vertical
scale is logarithmic, each division representing a tenfold increase or
decrease.
In
the physical evolution of the planet, the heavier elements became more
abundant as the solar system formed. The accompanying bar chart shows the
relative abundances of the most important elements. Note that the vertical
scale is logarithmic, each division representing a tenfold increase or
decrease.
The bar chart reveals the processes that synthesised heavier elements out of the hydrogen (H) and helium (He) of the Big Bang. Fusion in stars created more helium, skipped over lithium (Li), beryllium (Be) and boron (B) to carbon (C) and generated all the elements up to iron (Fe). Massive stars eventually explode as supernovas and their shockwaves make heavier elements than iron, but in very small amounts.
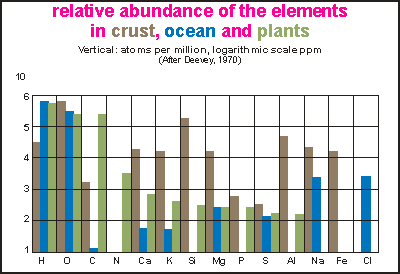 This
bar chart shows one division for each element horizontally and abundance
in a logarithmic scale, vertically. For each element, the concentrations
for land (brown), sea (blue) and plants (green) is shown. In fact, the
green data is the total of all life, but since the mass of plants far outweighs
that of animals, it can be considered plants alone. Note that the concentration
is in parts per million, counting atoms, not by weight. The top division
is 1E6 or 100%, the bottom line is 1 ppm.
This
bar chart shows one division for each element horizontally and abundance
in a logarithmic scale, vertically. For each element, the concentrations
for land (brown), sea (blue) and plants (green) is shown. In fact, the
green data is the total of all life, but since the mass of plants far outweighs
that of animals, it can be considered plants alone. Note that the concentration
is in parts per million, counting atoms, not by weight. The top division
is 1E6 or 100%, the bottom line is 1 ppm.